Sterilizing is the final step to ensure patients' and staff's safety before or after a procedure. An item is considered sterile if the probability that a single viable organism or virus is present on it is less than or equal to 10–6.
But why should we use steam sterilization?
For dental equipment that needs to be sterilized, steam sterilization is the safest and most preferable sterilization method as it damages the microorganisms of the cell that contain the protein.
And how does it work?
During the procedure, water is heated until the entire sterilization chamber is filled with steam, which usually cannot be higher than 100°C | 212 °F, however, due to the increased pressure the so-called 'tense steam', which contains a higher heat density, allows the required temperature of 135°C | 272°F.
1. Ventilation
Through the vacuum pump, the entire air is sucked out of the sterilization chamber and from the (inside of the) instruments. If this is not done correctly, the steam cannot occupy the chamber and items to guarantee the full sterilization
Modern sterilizers provide a so-called fractional vacuum process, that ensures that the chamber is evacuated multiple times to secure and prove the vacuum.
2. Sterilization
The sterilization process is divided into the actual organism killing time and the security time. The organism killing time is the time frame required to bond the protein of the microorganisms, while the security time provides an additional time frame for security reasons.
3. Drying phase
As already illustrated in chapter 3. manual disinfection & drying during the manual maintenance guide or chapter 2. automated Cleaning & Disinfection in the automated maintenance guide it is essential to dry off the equipment. Gladly this process is automatically included in most modern autoclaves - still it should always be confirmed that the drying cycle works properly.
During the drying phase the autoclave created another vacuum to clear out all the moisture.
Process:
a) Required equipment:
- Vacuum steam sterilizer according to EN13060 | ISO 17665-1
- Sterilization pouch according to ISO11607-1
- Sealing device for the above-mentioned pouch
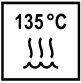 Please note that only items can be autoclaved if the below-mentioned symbol is printed on the product. Kindly note that all MK-dent handpieces can and must be autoclaved according to the instructions for use.
Please note that only items can be autoclaved if the below-mentioned symbol is printed on the product. Kindly note that all MK-dent handpieces can and must be autoclaved according to the instructions for use.
b) Ensure the following steps are executed properly:
1. Pack the handpiece into the sterilization pouch
Ensure the sterilization ouch is reasonably sized and well-fitting for the handpiece.

Handpieces must be individually packed, in addition, check and confirm that all seals are correct.
2. Sterilisation process
Place the sealed handpiece inside the autoclave and ensure the following process is executed properly:
- Fractionated pre-vacuum
- >4min with 135°C
- Execute the full drying cycle
The above instructions and the entire maintenance guide have been approved by the medical device manufacturer as appropriate for the preparation of a medical device
validated for reuse. The processor is responsible for ensuring that the processing actually carried out
achieve the desired result with the equipment, materials and personnel used in the processing facility. are for
Verification and/or validation and routine monitoring of the process required.
Storage
Store reprocessed instruments in a dry, dark and cool room, protected from dust and as germ-free as possible. Observe the expiry date of the sterile items.
That already completes the maintenance guide!
_Druckdaten_2022.png?height=120&name=2020x500_cm_ADX22_(Blende)_Druckdaten_2022.png)